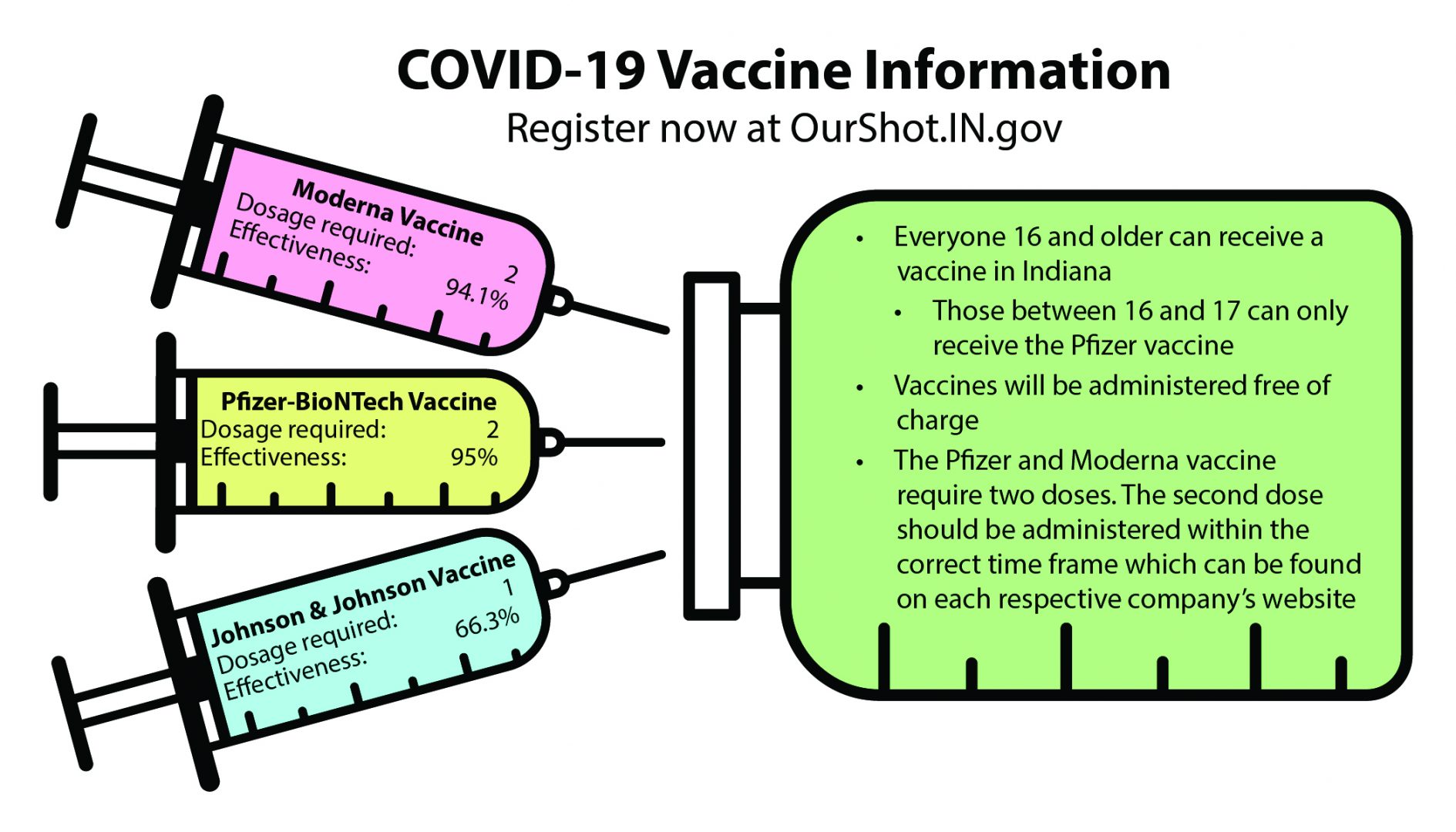
As of March 31, those who are 16 or older in the state of Indiana are eligible to receive the COVID-19 vaccine. According to University of Indianapolis Assistant Professor of Kinesiology and Public Health Kara Cecil, there are currently three vaccines available for individuals.
“Two of them [Pzfier and Moderna] are a two-shot series. The Johnson and Johnson is a single-shot vaccine,” Cecil said. “They have all gone through the standardized testing process that we [scientists] use for any flu vaccine or an MMR [measles, mumps, and rubella vaccine].”
According to Cecil, all three vaccines are similar, despite the number of shots. For the two-shot vaccines, Cecil said, the rate of protection from the first shot is around 80%, and after the second dose, it gives a marginal increase of protection against COVID-19.
“A lot of our vaccines actually are multiple shot series. Like the MMR is a good example,” Cecil said. “You’ll get those — a lot of those childhood vaccines that maybe people are a few years out from remembering having to get –– but many, many of those are multiple series of vaccines to get the immunity protection.”
Although there may be skepticism, Cecil said, all vaccines have been through multiple tests and are continually being monitored for any issues.
Associate Professor and Director of the Public Health Program Heidi Hancher-Rauch said that the rollout of these vaccines has been very positive.
“[It has been] really positive in terms of what we’re seeing in the vaccine’s ability to prevent infection from COVID in general, as well as prevent serious complications, hospitalization and death,” Hancher-Rauch said. “So really good news coming out about the vaccines overall. And so now the biggest push is we just need to get people vaccinated.”
According to Cecil, once vaccines are considered safe, they are released. If anything were to interfere with the vaccine’s safety, Cecil said, the distribution would stop, and there would be an investigation. She said ongoing, rigorous safety monitoring and evaluation continues for the COVID-19 vaccine, just as for any flu shot or allergy medication. Cecil said that no one is being injected with the live COVID-19 virus.
Hancher-Rauch said that if someone truly came down with the disease after vaccination, it was not the vaccine but that the person was exposed to the virus prior to vaccination, or that the vaccine had not yet had enough time for the antibodies that fight off the infection to develop.
Reactions to the vaccine have been reported, and Hancher-Rauch said these are the immune system responding to the vaccine, and this is how they want it to react. She said that those who receive the vaccine may develop a mild fever or sore arm or even chills, and that is OK.
“It doesn’t mean that you have developed COVID from it,” Hancher-Rauch said. “What it means is that your immune system is working as it should. It means that it is developing the antibodies. It is fighting off what it sees as the intruder, which is what the vaccine delivered. And it’s really preparing your body to fight off COVID if you are exposed to it in the future.”
Cecil and Hancher-Rauch both recommend that if there happen to be any other doubts about the vaccine, they do as much research as they can from reliable sources. Cecil said people can look for credible sites, such as .gov websites and the World Health Organization, to answer critical questions and can ask their primary care providers. Hancher-Rauch said that people can also look at sources such as the Centers for Disease and Control and Prevention and Dr. Anthony Fauci, or a personal physician.
Hancher-Rauch encourages students to obtain accurate information and then get vaccinated unless a physician tells them not to because of a possible allergic reaction or an underlying condition. She said if someone is hesitant because of uncertainty about trusting the science or because someone else has experienced a reaction, that person should do his or her own digging and critical thinking. She said to make sure the decision is based on accurate information.
“If we want to get back to normal, if we want to have a normal campus and we want to be able to just engage with each other like we have done in the past and really take advantage of all that our university has to offer, then we need people to get vaccinated,” Hancher-Rauch said.
In the latest development, the CDC and the FDA announced that there would be a pause in the distribution of Johnson and Johnson vaccines. The one-shot vaccine has been reported to have caused blood clots in six female patients, Cecil said. All the women were between the ages of 18 and 48, with the reactions showing up between days six and thirteen post vaccination, Cecil said.
According to Hancher-Rauch, the pause of the vaccine shows that there is a demonstration of protection for future individuals who may get the vaccine.
“When you look at the numbers, six people out of over seven million had that blood clot and come to find out they had a rare blood clotting disorder,” Hancher-Rauch said. “Just to double-check and make sure they [CDC] went ahead and pulled it to do a bit more research, mainly more for, I would say everybody’s confidence. When you do the statistics, it [is] less than one in a million chance [of getting a blood clot].”
Cecil and Hancher-Rauch both said that these adverse reactions are a risk within vaccines. In fact, Cecil said that 16.5 percent of people get a blood clot from contracting COVID-19 than from getting a blood clot from the vaccine, which is less than one in a million.
“Individuals will make the choice about the level of risk they’re willing to tolerate,” Cecil said. “Are you willing to understand the risk of one in a million, the odds of one in a million [of] getting a blood clot reaction, as opposed to 16 and a half percent having blood clots, post-COVID infection?”
The pause on the Johnson and Johnson vaccine was lifted on April 23 after scientific advisers came to the conclusion that the benefits of the vaccine outweigh the risks, according to The Associated Press. It was found that there were 15 vaccine recipients who developed a rare blood clot, resulting in three deaths, according to AP. Health officials decided that the J&J vaccine should resume as it is critical in fighting the COVID-19 pandemic and that the clots should be handled as a warning for younger women and their decision on whether or not to risk receiving the J&J vaccine or an alternative, according to AP.